Blog Post: The Importance of Medical Grade Inks
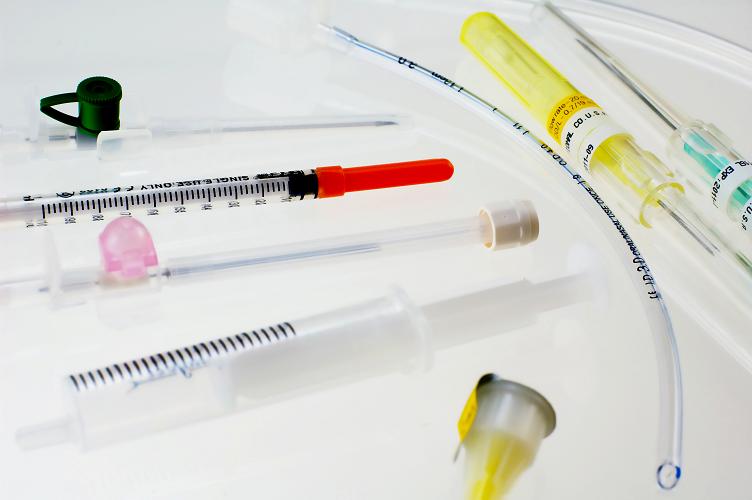
Medical grade inks used to mark medical devices should meet important safety standards. The United States Pharmacopoeia (USP) 30, NF 25, 2007 standard also known as Class VI is widely used to comply with stringent FDA rules for products that come in contact with the human body. This standard includes a full range of tests, procedures and industry best practices for all phases of pharmaceutical and medical device development and manufacturing.
The USP Class VI biological reactivity testing includes System Toxicity and Intracutaneous Toxicity tests which are used to establish the safety of a particular product. During these tests, the subject material and a negative control are injected into animals which are observed for a period of time. The animal's response to the material and the control substance are compared to determine if the test was passed. It is important that materials used for the manufacturing of medical devices pass these tests and are considered biocompatible in order to eliminate the risk of adverse reactions in patients.
TouchMark takes patient safety very seriously and only uses Class VI medical grade inks for printing on medical devices. Our inks go through these rigorous toxicity tests to make sure our customers meet or exceed FDA standards.
